I. Overview
The negative electrode material is a carrier of lithium ions and electrons during the charging process of the battery, and plays the role of energy storage and release. Among the battery cost, the anode material accounts for about 5%-15%, which is one of the important raw materials for lithium ion batteries.
The global lithium battery anode material sales volume is more than 100,000 tons, mainly in China and Japan. According to the current growth trend of new energy vehicles, the demand for anode materials will also continue to grow. At present, the global anode materials for lithium batteries are still dominated by natural/artificial graphite, and new anode materials such as mesocarbon microbeads (MCMB), lithium titanate, silicon-based anodes, HC/SC, and lithium metal are also growing rapidly.
As a carrier for lithium ion intercalation, the negative electrode material must meet the following requirements:
The insertion of lithium ions in the negative electrode matrix has an oxidation-reduction potential as low as possible, close to the potential of the metal lithium, thereby making the input voltage of the battery high;
A large amount of lithium in the matrix can undergo reversible insertion and deintercalation to obtain high capacity;
There is no or little change in the structure of the negative electrode during the insertion/deintercalation process;
The redox potential should be changed as little as possible with the insertion and removal of Li, so that the voltage of the battery does not change significantly, and a smoother charge and discharge can be maintained;
The intercalation compound should have good electronic conductivity and ionic conductivity, which can reduce polarization and enable high current charge and discharge;
The host material has a good surface structure and can form a good SEI with the liquid electrolyte;
The intercalation compound has good chemical stability over the entire voltage range and does not react with the electrolyte or the like after forming the SEI;
Lithium ions have a large diffusion coefficient in the host material, which facilitates rapid charge and discharge;
From a practical point of view, materials should be economical and environmentally friendly.
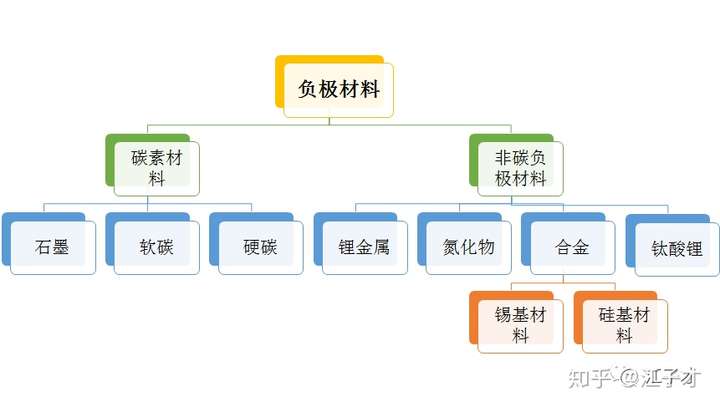
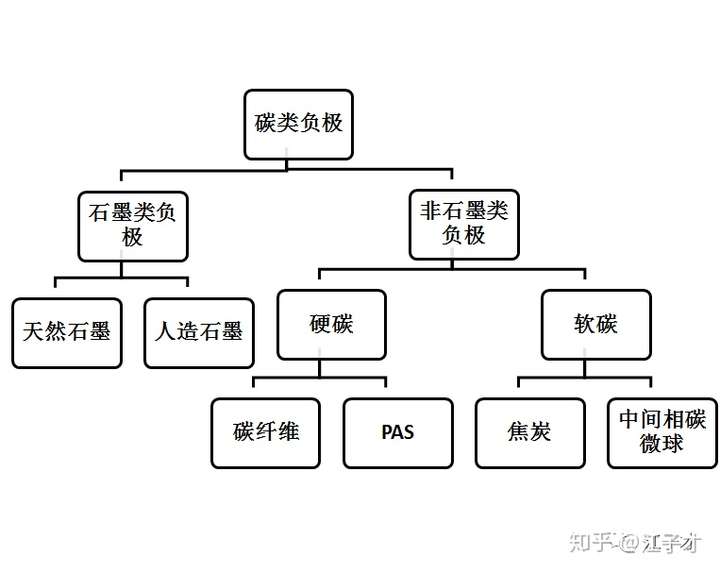
Two carbon anode material
The figure below shows the classification of common carbon anode materials.
2.1 Graphite anode
Graphite, English name graphite, graphite soft, slippery, is a non-metallic mineral, with high temperature resistance, oxidation resistance, corrosion resistance, thermal shock resistance, high strength, good toughness, high self-lubricating strength, heat conduction, electrical conductivity Unique physical and chemical properties such as high performance.

Graphite has many excellent properties and is widely used in metallurgy, machinery, electrical, chemical, textile, defense and other industrial sectors, such as graphite molds, graphite electrodes, graphite refractories, graphite lubricants, graphite seal materials. China is the country with the most abundant graphite reserves in the world, and is also the largest producer and exporter in the world, and plays an important role in the world graphite industry. According to the statistics of the Ministry of Land and Resources of China, China's crystalline graphite reserves are 30.85 million tons, basic reserves are 52.8 million tons; cryptocrystalline graphite reserves are 13.58 million tons, and basic reserves are 23.71 million tons. China's graphite reserves account for a large proportion of world reserves.。
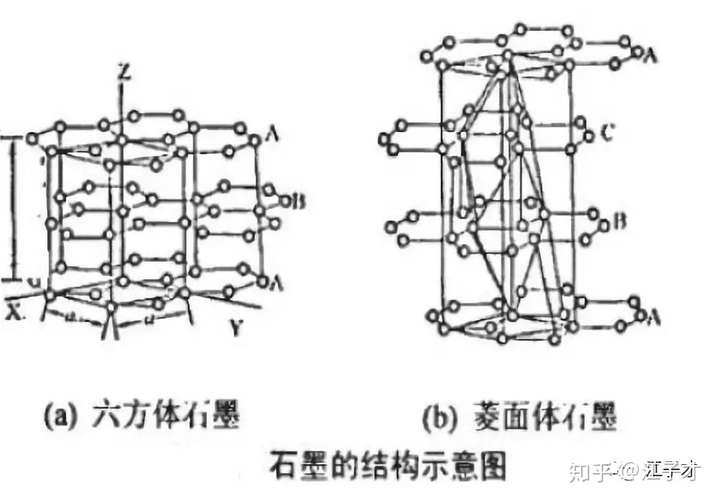
The ideal graphite has a layered structure, and the layer is formed by a carbon atom of SP2 to form a huge plane similar to a benzene ring. The carbon atoms between the planes of the layer are connected by a δ bond, and the bond length is 0.142 nm and the bond angle is 120°. There is also a large π bond connecting all carbon atoms between the layers. The interlayer is 0.3354 nm. Two crystal forms: hexagonal type-2H type (a) and water chestnut crystal system - 3R (b) Ø two crystal forms can be converted to each other: grinding and heating.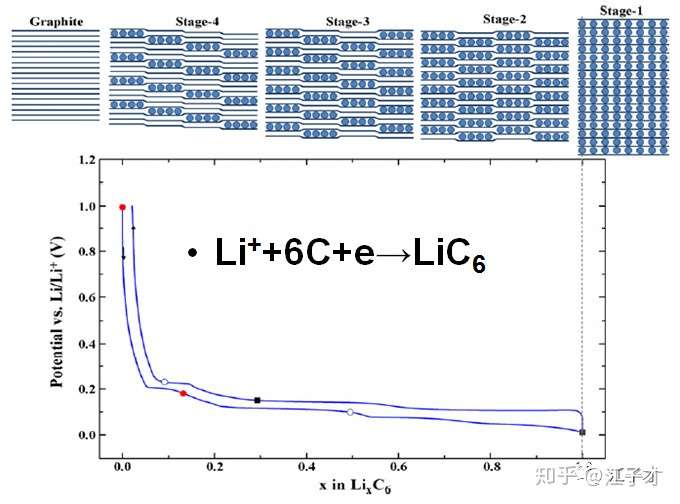
Lithium intercalation mechanism of graphite
The theoretical capacity of graphite is 372 mAh/g. Of course, only materials with very high degree of graphitization can reach this value. However, all carbon materials will have irreversible capacity loss due to side reactions during the first charge and discharge. As the potential of the negative electrode is lowered, the composition stops in the electrolyte until a stable passivation film (SEI) is formed on the surface of the negative electrode. Four voltage platforms appear in the first discharge (see the figure below), where A is the formation of SEI, and most of the graphite capacity is in the range of 0.3~0.005V. In addition to A, different voltage platforms correspond to different lithium-incorporating states, which are called fourth-order and third-order compounds, respectively. Finally, LiC6 is formed, reaching a theoretical capacity of 372 mAh/g, and the interplanar spacing becomes 0.37.
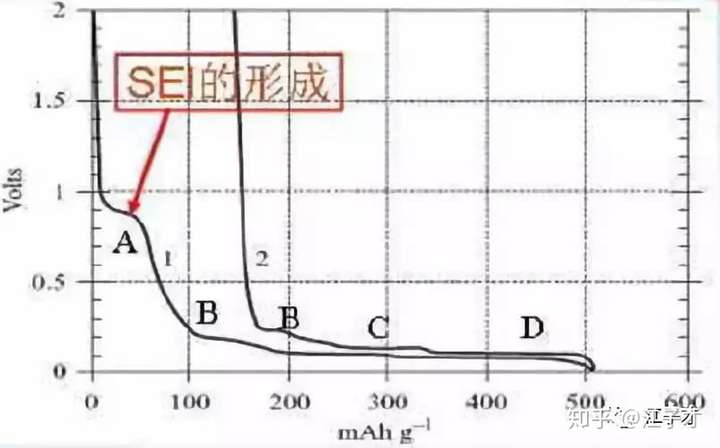
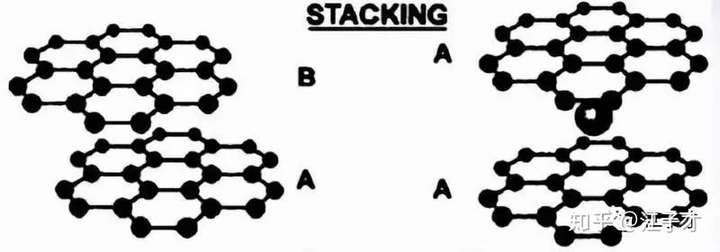
The arrangement of the graphite LiC6 ink sheets in the fully inserted lithium state is changed (as shown in the following figure): from ABABAB ... to AAAA ... arrangement. Some artificial graphite is difficult to convert and has a low capacity.
Graphite is mainly divided into natural graphite and artificial graphite. Natural graphite needs to be treated as a negative electrode for lithium ion batteries, such as our common oxidation treatment and mechanical grinding. The artificial graphite is converted from organic matter (gaseous, liquid, solid) to graphite. The specific operation method can be Baidu.。
Having said that, of course, because he used the most. Of course, as a negative electrode material, graphite also has many disadvantages, such as the low potential of graphite, forming an interface film with the electrolyte, and easily causing lithium deposition; the ion migration speed is slow, so the charge and discharge rate is low; the layered structure of graphite is in lithium. About 10% of deformation occurs during ion insertion and deintercalation, affecting the cycle life of the battery.
2.2 Non-graphite anodes as above
Non-graphite anodes mainly have hard carbon and soft carbon. Soft carbon, which is easy to graphitize carbon, refers to amorphous carbon that can be graphitized above 2000 °C, with low crystallinity, small grain size, large interplanar spacing, and compatibility with electrolyte. it is good. However, the first charge and discharge irreversible capacity is high, the output voltage is low, due to his performance, generally do not directly make negative electrode materials, is the raw material for the manufacture of natural graphite, common petroleum coke, needle coke and so on.

Hard carbon, which is also difficult to graphitize carbon, is a pyrolytic carbon of high molecular weight polymers. Such carbon is difficult to graphitize at a high temperature of 3000 °C. Hard carbon is resin carbon (such as phenolic resin, epoxy resin, polydecyl alcohol, etc.), organic polymer pyrolysis carbon (PVA, PVC, PVDF, PAN, etc.), carbon black (acetylene black); is conducive to the insertion of lithium without It will cause significant expansion of the structure and has good charge and discharge cycle performance.
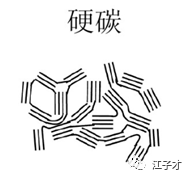
The hard carbon capacity is greater than the theoretical capacity of conventional carbon materials, high rate, cycle performance, and excellent safety performance, but the first effect is low, about 85%, the full battery voltage platform 3.6V is lower than the graphite 3.7V, and the cost is high. The improvement ideas are mainly to improve the first effect (reduced specific surface area, form more regular hard carbon; surface coating, control SEI formation); improve material yield and reduce cost.

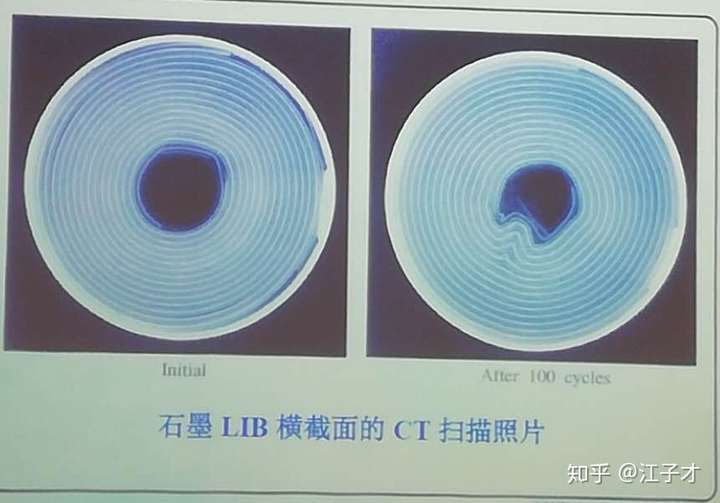
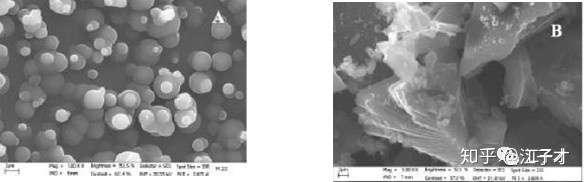
From the comparison of the pictures, HC is more stable than conventional graphite-based anode materials.