Fortunately, due to the previous work, there are hundreds of batteries that have been dismantled in my hands. Lithium iron phosphate, ternary, soft packs, and cylindrical batteries are involved. Today, some pictures left are dismantled and shown to everyone. At a glance, what is the state of the internal pole piece of the battery. The following pictures are based on the negative electrode state of a certain battery. After the lithium ion enters, the graphite negative electrode will gradually turn golden yellow, while the positive electrode is difficult to observe by color change. We usually judge the battery charge and discharge mechanism and the quality of the battery by disassembling the battery and observing the interface state of the negative electrode

When SOC=7%, it was found that there was a grain in the center of the electrode, and the electrode did not change color

. SOC = 14%, and the texture at the center of the electrode can be seen. Slightly discolored.

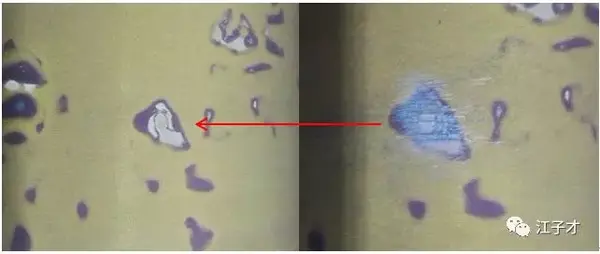
SOC=26%, slightly turned blue.

SOC=53.9%, it turns blue, and the texture of the bubble can be seen

SOC=70.0%, which becomes a dark purple reddish color, and can see black uncharged areas. No lithium ion precipitation was observed.

SOC = 81.5%, it turns golden, and you can see the black uncharged area. Can see the phenomenon of lithium deposition in white.
According to the above picture, the state of the electrode does not change much in the stage where the SOC is less than 50%.
The change of the electrode can be observed from 50% or more. According to the picture 4, it is found that a large number of bubbles are generated at around 3.6V. It can be inferred that a very large change point has occurred between FIG. 5 and FIG. 6 in particular.
The SOC has only changed by 10%, but the state of the electrodes is completely different.

When the SOC=100%, the battery is in the state of the pole piece.
Most of the white part is lithium. The surface of the pole piece below the white portion was not turned into gold, which indicates that lithium ions were not embedded in the anode material. In short, this part of the negative electrode material hinders the intercalation of lithium ions, so that lithium ions are deposited on the surface. It is speculated that the binder (CMC, SBR) may be unevenly distributed on the surface of the electrode and may be encapsulated by the carbon of the negative electrode material, so that lithium ions cannot be embedded. When SOC=100%, the black dot portion is mostly an uncharged region. More black spots are an important cause of low battery capacity. The main factors of black spots are the uniformity of the slurry, the control accuracy of the coated surface density, the tension control of the winding, and the formation of gas.
